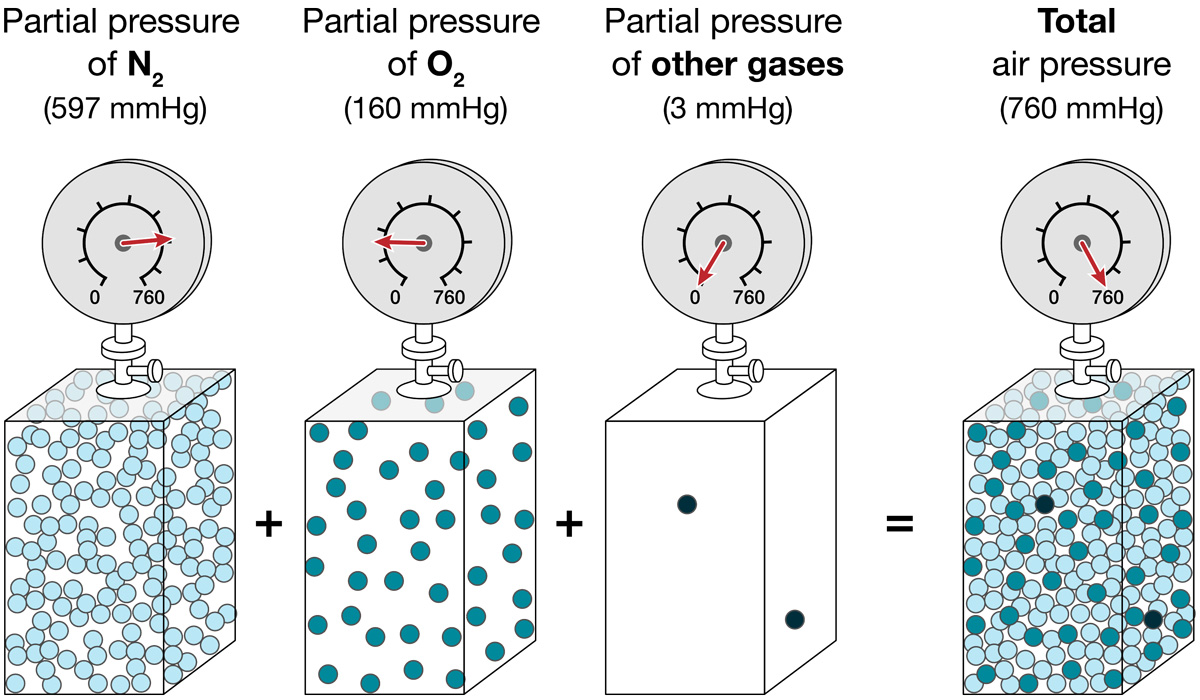
Oxygen in the Atmosphere:
Partial Pressure
Even though the percentage of oxygen doesn’t change with elevation, the pressure of oxygen does.
Air is a mixture of molecules from different gases. The pressure of all these molecules together is the total air pressure.
The pressure of the molecules of a specific gas is its partial pressure. So, the partial pressure for oxygen is the pressure exerted by only the oxygen molecules in the air.
To calculate the partial pressure of a specific gas, multiply the total air pressure by the proportion of the air made up of that gas. For example, air is 21% oxygen. So, the partial pressure of oxygen (written as PO2) is 21% of the total air pressure.
At sea level, where the total air pressure is 760 mmHg, the partial pressure of oxygen is 160 mmHg.