Oxygen in the Atmosphere:
Air Pressure
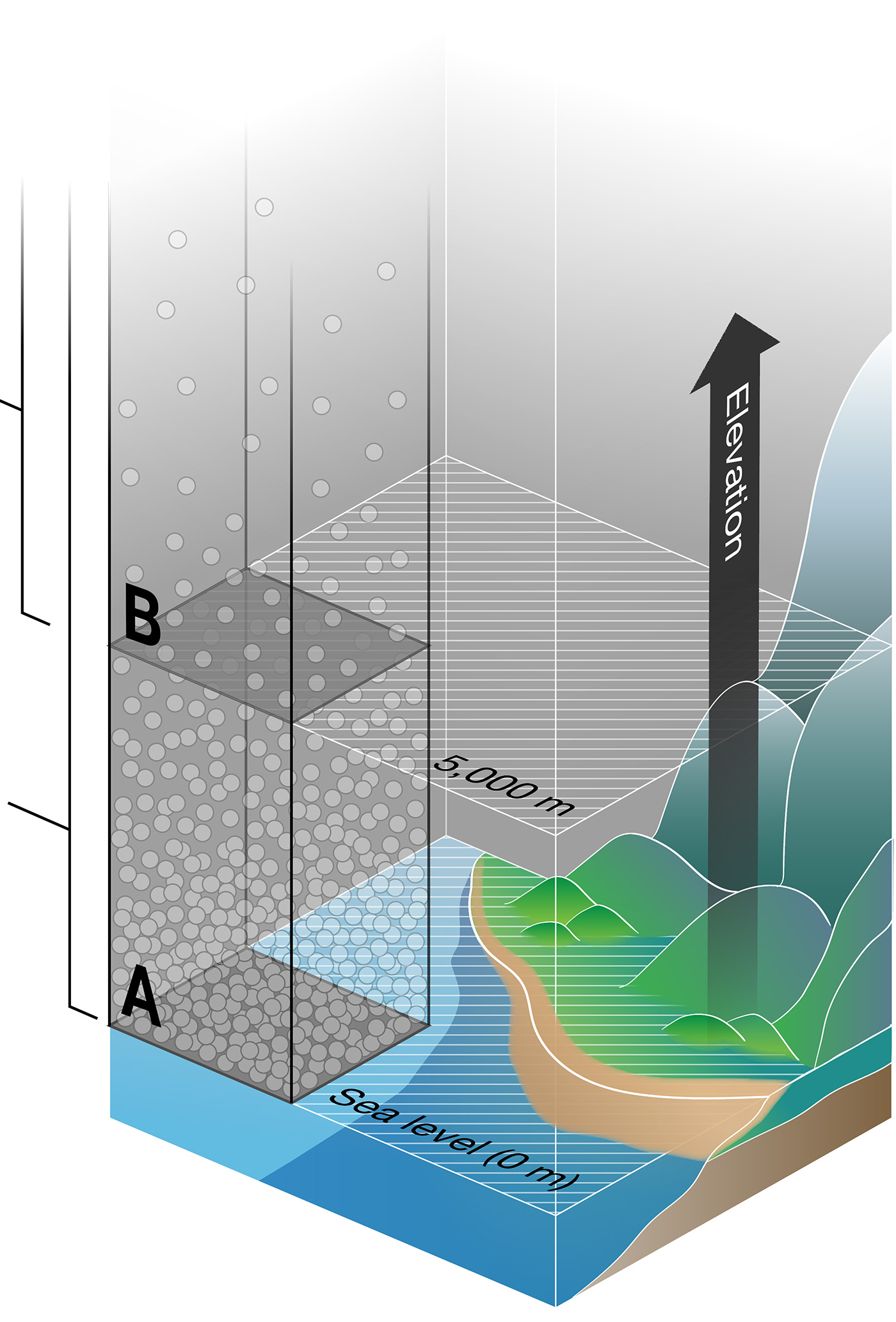
It might feel like there's nothing in the air around us, but air is a mixture of gas molecules. Due to the pull of Earth's gravity, there are more gas molecules per unit area at lower elevations than at higher elevations. The weight (or force) of all the gas molecules (white dots in Figure 1) above a unit of area is called the air pressure.
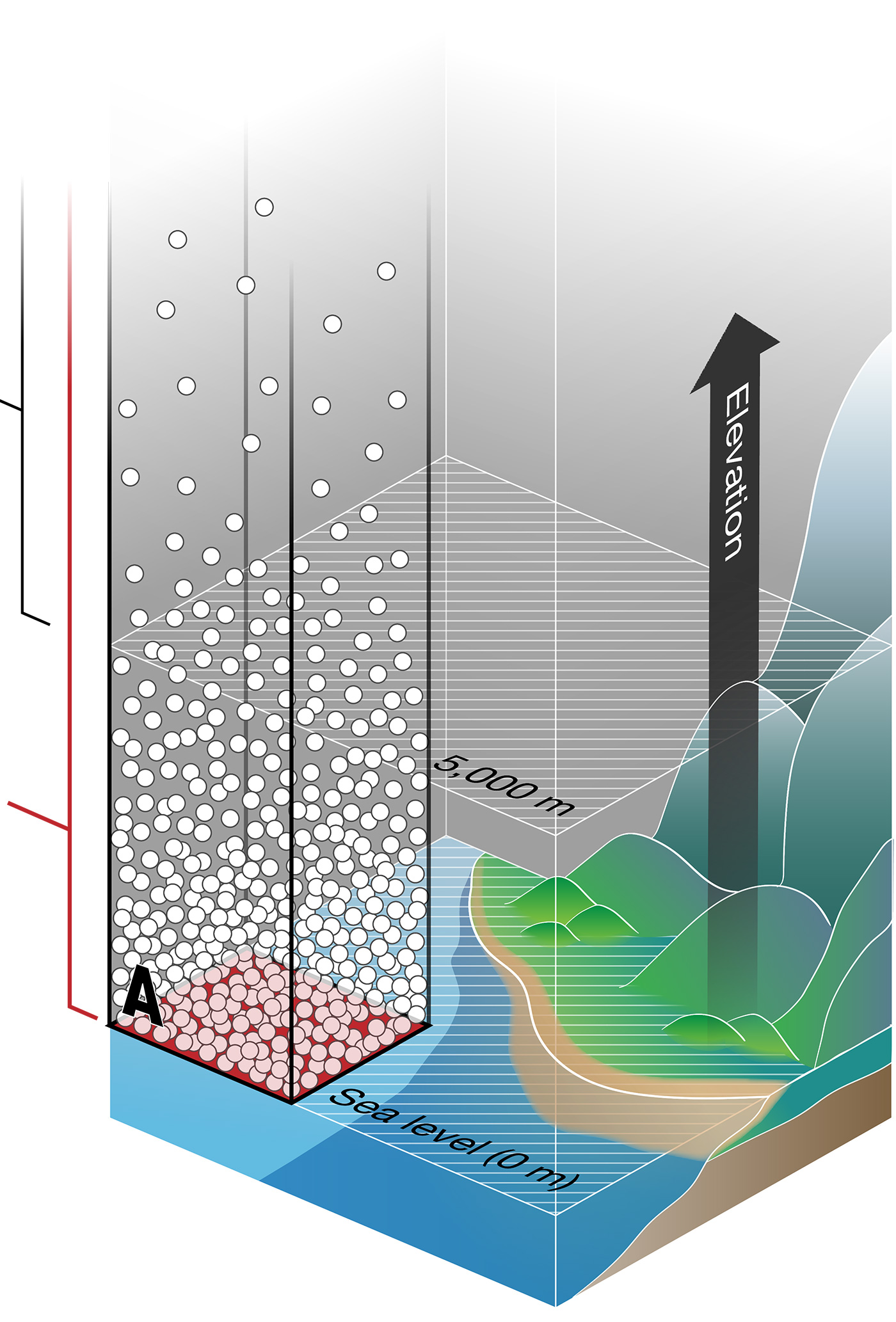
At sea level (Area A in Figure 1), many gas molecules are pressing down on a given unit of area. The average air pressure is 760 mmHg (101 kPa).
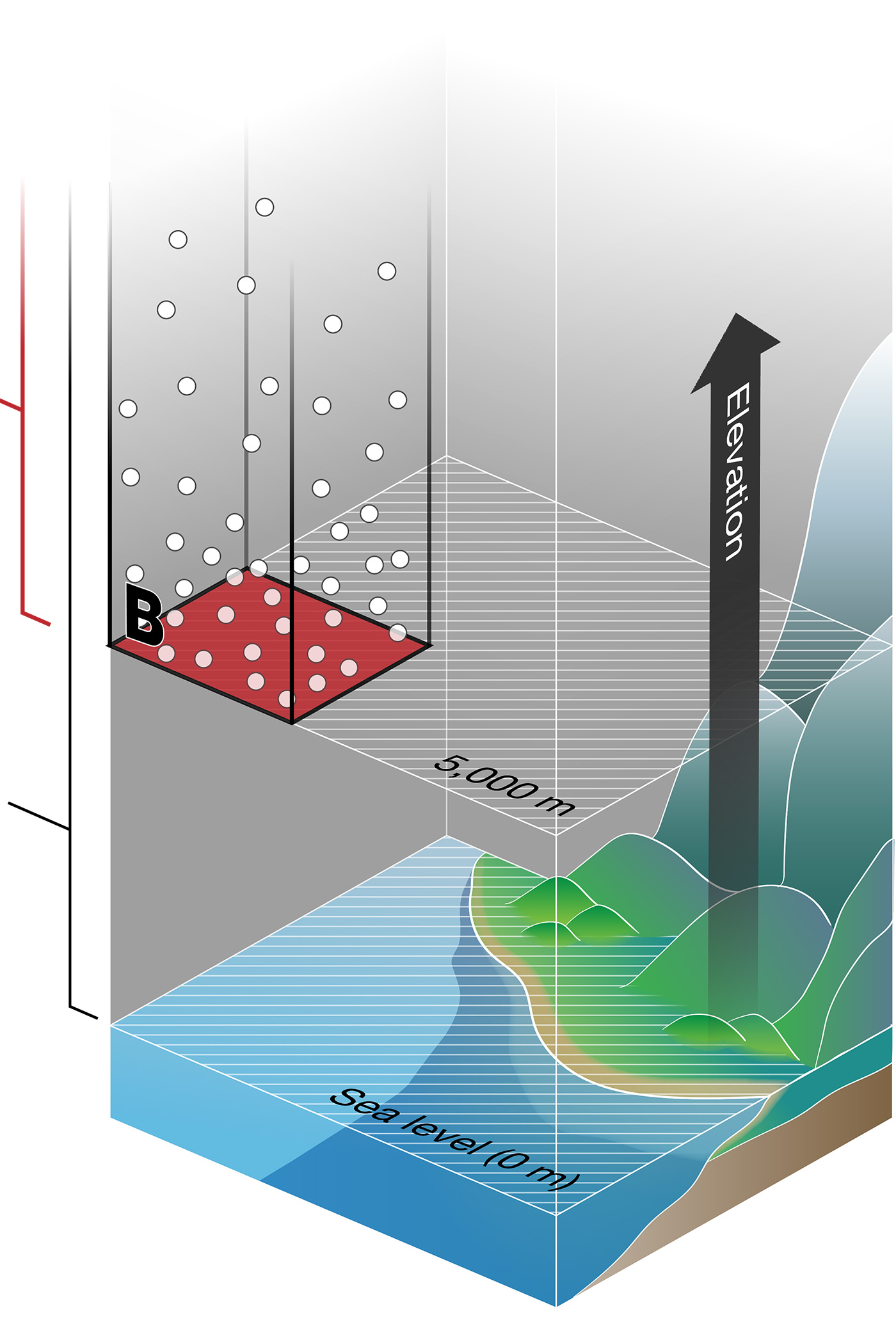
At 5,000 m above sea level (Area B in Figure 1) — the same elevation at which the ALMA antenna array is located — fewer gas molecules are pressing down on the same unit of area. So, the average air pressure is lower: 420 mmHg (56 kPa).
In summary, the higher the elevation, the lower the air pressure.
Pressure can be measured in many different units. The unit mmHg, or millimeters of mercury, is the most common unit of pressure used in medicine. The unit kPa, or kilopascals, is 1,000 pascals (Pa): a unit of pressure based on the metric system.
Other units of pressure include millibars (mbar), torrs, atmospheres (atm), and pounds per square inch (psi). The average air pressure at sea level can be written as 760 mmHg, 101 kPa, 1,013 mbar, 760 Torr, 1 atm, or 14.7 psi.
Different units of pressure may be used for different purposes. For example, air pressure in car or bike tires is usually reported in psi. The air pressure at sea level (14.7 psi) is about half of the air pressure in most car tires.
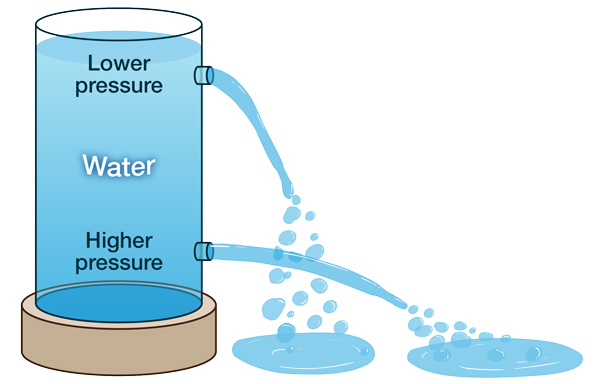
To help visualize how elevation affects pressure, imagine a tall container filled with water. If you poke a hole just below the top of the container, water will dribble out slowly. But if you poke a hole at the bottom of the container, water will rush out more quickly.
Why does this happen? At the bottom of the container, the weight of all the water molecules above causes higher pressure, resulting in a stronger rush of water through the hole. Similarly, air pressure is higher at lower elevations (closer to sea level), due to the weight of all the gas molecules above.