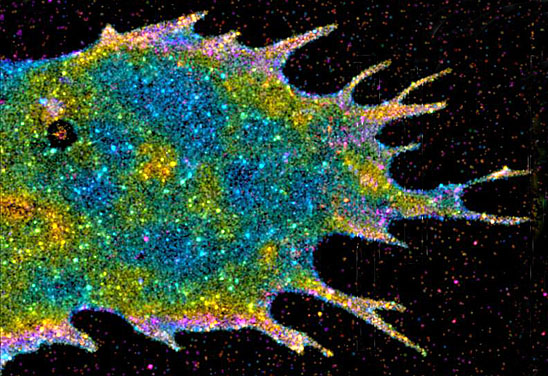
Several groups have expanded the power of super-resolution optical imaging with tools that allow scientists to see a once-impossible level of detail with a light microscope. The first generation of these microscopes took extraordinarily detailed images, permitting scientists to pinpoint the locations of individual proteins inside a cell. But the imaging process was too slow to capture molecules in motion.
Janelia Farm group leader Mats Gustafsson, the inventor of structured illumination microscopy, has enhanced the approach so that the microscope can capture up to 11 images per second: enough to create movies of proteins zipping along their molecular tracks to transport cellular supplies.
Janelia Farm group leader Harald Hess, coinventor of another superresolution imaging technique known as photoactivatable light microscopy (PALM), has introduced a third dimension to his cellular portraits. The new version of PALM, interferometric PALM, borrows from a technique widely used in industry to study minutiae such as subtle irregularities on the surface of a computer chip. The technology produces the best three-dimensional resolution ever seen with an optical microscope and will help reveal how molecules organize themselves into the structures and signaling complexes that drive cellular functions.
A third advance comes from Bernardo Sabatini, an HHMI investigator at Harvard Medical School. Sabatini dramatically improved the spatial resolution of two-photon microscopy, a leading method for seeing beneath the surface of the brain, by combining it with the imaging strategy that underlies a superresolution approach known as stimulated emission depletion. The new technique brings into focus once blurry features on cells too deep within a tissue to be seen with other forms of light microscopy—meaning scientists can now see tiny details, such as the connections between nerve cells, inside relatively intact samples of brain tissue.
Signals race from neuron to neuron in a matter of a millisecond, so watching that communication take place demands imaging technologies that can keep up the pace. Rafael Yuste, an HHMI investigator at Columbia University, has greatly sped up imaging by combining elements from a two-photon laser microscope with a tool often used to manipulate light waves to create holograms. The device splits a single laser beam into a pattern of light, so that collecting an image does not require waiting for a single laser to scan a whole field of cells. The laser-splitting technology also allows researchers to manipulate living cells, stimulating neuron firing in any pattern in space or time.